Νέα 2011
Deciduous Teeth
- Written by: Dr. Koliakos

Biohellenika today gives a second chance to children that have not cryopreserved stem cells from the umbilical cord blood to store stem cells from deciduous teeth, which from the age of 5-6 year old are replaced.
These cells today are in experimental studies for regenerative medicine.
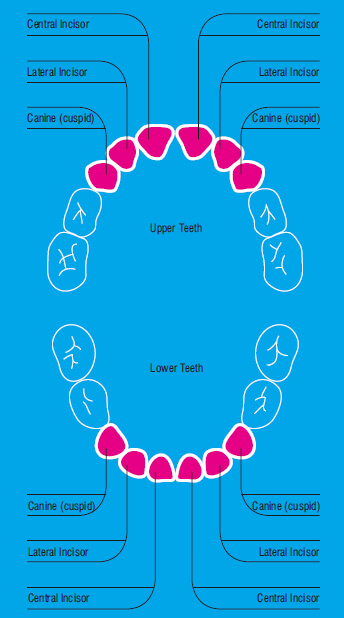
The aim of this service is the collection and cryopreservation of the valuable biological source of stem cells for the children that have not collected their stem cells during birth or that have been used for therapeutic purposes. The deciduous teeth that are used for this purpose are highlighted in the following diagram in red.
The service also concerns adults that want to use their wisdom teeth as a source of collection, process and cryopreservation of stem cells for therapeutic use. Furthermore teeth that are extracted for orthodontic purposes can be used for the isolation of stem cells.
The discovery that was done in 2003 from a scientific team of the Department of National Institute of Health of the United States (N.I.H.) gives the opportunity to parents that lost the chance to store their childrens’ stem cells during birth to ask for the preservation of this valuable material that is found in deciduous teeth.
Types of stem cells that are found in the dental pulp.
The deciduous teeth inside contains the dental pulp, which is rich in stem cells. The discovery of these cells open a new field of research, that explores the possibilities of use these cells in therapeutic applications in the near future, in parallel or complementary to those of the umbilical cord stem cells.
Τhe pulp of deciduous teeth contains:
Odontoblasts, the cells which create the pulp and the dentine of the tooth
Mesenchymal stem cells which can be implanted into any other organ or tissue and reinforce its defense and regenerating function
Endothelial cells and also migrating blood cells.
Today is generally acceptable, that stem cells derived from deciduous teeth can have in the near future clinical applications similar to those of the stem cells of the bone marrow and the umbilical cord blood.
Diseases that are intended to be treated with dental pulp stem cells are:
Dental diseases
Pulp regeneration
Development a whole new viable teeth
Dentin regeneration, by fixing the caries
Tissue regeneration
Improvement of cardiac function after heart attack, by stem cell implantation into the infarcted area
Treatment of neurodegenerative diseases
Osteoarthritis, cartilage and fascia regeneration
Bone fracture healing
Use as grafts in Maxillofacial Surgery of jaw or bone defects
MOLECULAR TEST FOR THE Human Papillomavirus – HPV
- Written by: Dr. Koliakos
 WHY IS THE VIRUS DANGEROUS?
WHY IS THE VIRUS DANGEROUS?
- Cervical carcinoma is the second most common type of cancer in women genital system, after ovary cancer.
-
HPV infections is the main cause of cervical cancer.
-
Nearly 471,000 new cases are reported annually, and 233,000 deaths [1,2]. In Europe approximately 60,000 new cases and nearly 30,000 deaths are recorded annually, while in Greece 8,0000 new cases are reported annually [3].
HOW IS THE VIRUS TRANSMITTED?
- HPV infection is one of the most common sexually transmitted diseases.
- Approximately 75-80% of women and men are infected with HPV at some time over the course of their lives.
- The frequency peak for detectable HPV infections lies in the age group between 20 and 25 years.
- An HPV infection is in most cases eliminated by the body’s immune system over a time period of about 8 to 14 months.
- In most cases the infection is asymptomatic and the carrier does not know that she/he has been infected by the virus.
- Exposure to HPV does not necessarily mean malignancy occurrence [5-9].
WHICH VIRAL TYPES ARE KNOWN TODAY?
- Well over 100 HPV types are known thus far, divided in high and low risk. The classification is made according to their propensity to trigger cancer growth [10].
- High-risk types cause precancerous stages (dysplasias, cervical intraepithelial neoplasias, CIN) and cancer. These are 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73 and 82. They are identified in 99.7% of all cases of cervical carcinoma. A majority of cervical carcinoma cases (approx. 70%) are triggered by just two hr-HPV types: HPV type 16 (53.5%) and HPV type 18 (17.2%)
- The ability of the virus to create tumor is increased, when multiple infections are present, with different HPV types.
- The most common low risk HPV types are 6, 11, 40, 42, 43 και 44 [11].
MOLECULAR TESTING ADVANTAGES
Molecular test, detection and genotyping of the virus is the most modern and reliable diagnosis method. It should be applied in combination with annual PAP-test in women of over of 30 years of age. Molecular testing is necessary, since PAP-test can detect only cervical damages and not the cause of the cancer.
Therefore, a molecular test that can quickly and reliably detect multiple HPV genotypes in cervical smear becomes a necessity. Early detection of the virus and monitoring of the infection is important, in order to avoid cancer. Negative result deriving from a molecular test offers the possibility of vaccinations against high risk HPV in women of all age.
Biohellenika S.A. offers the service of molecular detection and genotyping of HPV with the method of DNA microarrays (PapilloCheck – CE-IVD).
PapilloCheck is considered the most indicated way of detection and genotyping of HPV because:
- PapilloCheck is based on the most advanced diagnostic technologies of Molecular Biology. In specific, it is based on HPV-DNA hybridization on DNA-chips (Biochips). It is the only system based on DNA-MicroArrays.
- This technology allows for fast, reliable and highly sensitive (98%) detection of multiple HPV infections. In contrast, other methods fail to detect accurately multiple (over two) infections.
- Papillocheck is suitable for detection and genotyping of 24 viral types, 18 of which are classified as high risk and six as low risk, as already mentioned.
- Papillocheck is one of the few methods for HPV genotyping that is certified in the European Union (CE) as an in vitro diagnostic (IVD) for the qualitative type-specific identification of 24 human papillomavirus types from a cervical smear [12].
SAMPLING
HPV test is applied in cervical smear, collected with a special sterilized swab, offered for free by Biohellenika (after communication). The swab must be sent in our labs within 48 hours, while, until then, it is stored at 4°C.
References
1. Parkin D.M. et al. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55(2):74-108.
2. Ferlay J. et al. GLOBOCAN 2002: Cancer Incidence, mortality and prevalence worldwide, version 2.0 IARC; Cancer Bases No. 5, Lyon, IARC, 2004.
3. Boyle P. et al.; Cancer incidence and mortality in Europe, 2004;Ann. Oncol. 2005, 16(3):481-88.
4. Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (GEKID) in Zusammenarbeit mit dem Robert Koch-Institut (RKI); Krebs in Deutschland, Häufigkeiten und Trends; 5. überarbeitete, aktualisierte Ausgabe, 2006.
5. Ho G.Y.F. et al. Natural history of cervicovaginal papillomavirus infection in young women; N. Engl. J. Med. 1998, 338:423-428.
6. Bosch F.X., de Sanjosé S.; Chapter 1: Human papillomavirus and cervical cancer - burden and assessment of causality; J. Natl. Cancer Inst. Monogr. 2003, 31:3-13.
7. Winer R.L., Lee S.K., Hughes J.P., Adam D.E., Kiviat N.B., Koutsky L.A. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students; Am. J. Epidemiol. 2003, 157:218-226.
8. Brown D.R. et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women; J. Infect. Dis. 2005, 191:182-192.
9. Evander M. et al. Human papillomavirus infection is transient in young women: a population-based cohort study; J. Infect. Dis. 1995, 171:1026-1030.
10. Muñoz N., Bosch F.X., de Sanjosé S., Herrero R., Castellsague X., Shah K.V., Snijders P.J., Meijer C.J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group: Epidemiologic classification of human papillomavirus types associated with cervical cancer; N. Engl. J. Med. 2003, 348:518-527.
11. Muñoz N., Bosch F.X., Castellsagué X., Diaz M., de Sanjosé S., Hammouda D., Shah K.V., Meijer C.J. Against which human papillomavirus types shall we vaccinate and screen? The international perspective; Int. J. Cancer 2004, 111:278-285.
12. Dalstein V., Merlin S., Bali C., Saunier M., Dachez R. and Ronsin C. Analytical evaluation of the PapilloCheck test, a new commercial DNA chip for detection and genotyping of human papillomavirus. J. Vir. Methods 2009,156:77-83.
Infertility
- Written by: Dr. Koliakos

KARYOTYPE AND FISH-INFERTILITY
Biohellenika offers a complete testing program related to prenatal and postnatal control as well as tests related to infertility. These tests include karyotype and FISH to detect abnormalities of the chromosomes and tests for cistic fibrosis, thrombofilia, viral, parasitic and bacterial infections. The samples testes are: amniotic fluid, chorionic villi, material of miscarriages, fetal blood and peripheral blood.
Indications of prenatal screening include:
- Maternal age >35
- Ultrasound fetal abnormalities (eg indications for Down Syndrome)
- Increased risk of abnormalities portrayed in the biochemical control of the 1rst and second trimester.
- Parents who are carriers of chromosomal abnormalities
- Previous birth of a child with chromosomal abnormality
- Intrauterine death
- Determination of the sex in sex-linked diseases
- Spontaneous miscarriage
- Increased anxiety of the mother
Indications of postnatal control include:
- Infertility
- Recurrent miscarriages
- Medical history of intrauterine death or of previous child with birth defects.
- Apparent/visible congenital abnormalities
- Absence or delay of the development of the characteristics of the sex.
- Primary amenorrhea
The examinations of infertility include:
- FV-Leiden, F 2 (prothrombin), homocystinemia (MTHFR)
- Viral infections (herpes simplex, cytomegalovirus)
- Chlamydia, mycoplasma, naiseries
- Toxoplasma, B-streptococcus
The laboratories of biohellenika are accredited by ESYD (National Accreditation System). All tests are performed by qualified molecular biologists and evaluated by physicians specialized in Genetic Centers abroad with which they maintain partnerships. Apart from the tests mentioned above, other more specific tests can be conducted upon request. The information provided is valuable for the prognosis of the person with the abnormality, its relatives, future pregnancies and future generations.

Infertility tests
Biohellenika has built an up-to-date molecular analysis laboratory, which is capable of testing man and woman infertility. In our laboratory, microbial agents related to genital infections, such as Chlamydia, Toxoplasma, Ureoplasma and other microbes as well as viruses such as HPV, are being analyzed. These tests are conducted with the method of Real Time PCR (RT-PCR) and detect the very own DNA of the pathogens, which means their presence in the genital system at the moment of sampling. RT-PCR is a more reliable method, compared to antibody detection, which in some viral infections may take months. Hence false negative results are avoided and treatment as well as suitability of the medicine can be evaluated.
Thrombophilia tests
In cases of repeated miscarriages, pathologic genes responsible for blood coagulation may be triggered and therefore should be tested. These tests are very important, because they protect the mother from thrombotic cases during pregnancy, labor or even later.

Cardiovascular diseases tests
Patients with family history of heart attacks in early age are tested for carrying pathologic genes, associated with cholesterol metabolism and lipids in general, as well as for factors that may cause cardiovascular diseases. The “gene profile” of these patients can provide them with important information about their future. This information in combination with appropriate instructions and medical treatment can result in avoidance of life threatening cases.
So, Biohellenika offers the possibility of detecting the predisposition for cardiovascular diseases, which include a series of complicated damages not only in the heart, but also in the vessels. Such diseases are the major death cause in the advanced countries. Nowadays, the genetic factors that partake in thrombosis and arterial pressure rise mechanisms are known. Some of the diseases associated with pathologic genes are the following:
|
Diseases |
|
Severe vascular thrombosis |
|
Coronary disease |
|
Acute heart attack |
|
Hypertension |
|
Hereditary thrombophilia |
|
Thromboembolic disease |
|
Increased cholesterol levels in plasma |
Ρrenatal / Ρostnatal control
- Written by: Dr. Koliakos
New!
Biohellenika offers a complete testing program related to prenatal and postnatal control as well as tests related to infertility. These tests include karyotype and FISH to detect abnormalities of the chromosomes and tests for cistic fibrosis, thrombofilia, viral, parasitic and bacterial infections. The samples testes are: amniotic fluid, chorionic villi, material of miscarriages, fetal blood and peripheral blood.
Indications of prenatal screening include:
Ø Maternal age >35
Ø Ultrasound fetal abnormalities (eg indications for Down Syndrome)
Ø Increased risk of abnormalities portrayed in the biochemical control of the 1rst and second trimester.
Ø Parents who are carriers of chromosomal abnormalities
Ø Previous birth of a child with chromosomal abnormality
Ø Intrauterine death
Ø Determination of the sex in sex-linked diseases
Ø Spontaneous miscarriage
Ø Increased anxiety of the mother
Indications of postnatal control include:
Ø Infertility
Ø Recurrent miscarriages
Ø Medical history of intrauterine death or of previous child with birth defects.
Ø Apparent/visible congenital abnormalities
Ø Absence or delay of the development of the characteristics of the sex.
Ø Primary amenorrhea
The examinations of infertility include:
Ø FV-Leiden, F 2 (prothrombin), homocystinemia (MTHFR)
Ø Viral infections (herpes simplex, cytomegalovirus)
Ø Chlamydia, mycoplasma, naiseries
Ø Toxoplasma, B-streptococcus
The laboratories of biohellenika are accredited by ESYD (National Accreditation System). All tests are performed by qualified molecular biologists and evaluated by physicians specialized in Genetic Centers abroad with which they maintain partnerships. Apart from the tests mentioned above, other more specific tests can be conducted upon request. The information provided is valuable for the prognosis of the person with the abnormality, its relatives, future pregnancies and future generations.
ecard2012_en
- Written by: Dr. Kouzi
|
Current Stem Cell Applications |
Amegakaryocytosis Aplastic Anemia (Severe) Blackfan-Diamond Anemia Congenital Cytopenia* Congenital Dyserythropoietic Anemia Dyskeratosis Congenita Fanconi Anemia Paroxysmal Nocturnal Hemoglobinuria (PNH) Pure Red Cell Aplasia
Beta Thalassemia Major Sickle Cell Disease
Familial Erythrophagocytic Lymphohistiocytosis Hemophagocytosis Langerhans Cell Histiocytosis (Histiocytosis X)
Chronic Granulomatous Disease Congenital Neutropenia Leukocyte Adhesion Deficiency Severe Combined Immunodeficiencies (SCID) including: Adenosine Deaminase Deficiency* Bare Lymphocyte Syndrome Chediak-Higashi Syndrome* Kostmann Syndrome Omenn Syndrome Purine Nucleoside Phosphorylase Deficiency Reticular Dysgenesis Wiskott-Aldrich Syndrome X-Linked Lymphoproliferative Disorder
Adrenoleukodystrophy Fucosidosis Gaucher Disease* Hunter Syndrome (MPS-II) Hurler Syndrome (MPS-IH) Krabbe Disease Lesch-Nyhan Syndrome Mannosidosis* Maroteaux-Lamy Syndrome (MPS-VI) Metachromatic Leukodystrophy Mucolipidosis II (I-cell Disease)* Neuronal Ceroid Lipofuscinosis (Batten Disease)* Niemann-Pick Disease* Sandhoff Disease* Sanfilippo Syndrome (MPS-III) Scheie Syndrome (MPS-IS) Sly Syndrome Tay Sachs* Wolman Disease
Acute Biphenotypic Leukemia* Acute Lymphocytic Leukemia (ALL) Acute Myelogenous Leukemia (AML) Acute Undifferentiated Leukemia* Adult T Cell Leukemia/Lymphoma Chronic Lymphocytic Leukemia (CLL) Chronic Myelogenous Leukemia (CML) Hodgkins Lymphoma Juvenile Chronic Myelogenous Leukemia (JCML) Juvenile Myelomonocytic Leukemia (JMML) Myeloid/Natural Killer (NK) Cell Precursor Acute Leukemia Non-Hodgkins Lymphoma Polymphocytic Leukemia
Acute Myelofibrosis* Agnogenic Myeloid Metaplasia (Myelofibrosis)* Amyloidosis Chronic Myelomonocytic Leukemia (CMML) Essential Thrombocythemia* Polycythemia Vera* Refractory Anemias (RA) including: Refractory Anemia with Excess Blasts (RAEB) Refractory Anemia with Excess Blasts in Transformation (RAEB-T) Refractory Anemia with Ringed Sideroblasts (RARS)
Multiple Myeloma Plasma Cell Leukemia Waldenstroms Macroglobulinemia Other Inherited Disorders Cartilage-Hair Hypoplasia Congenital Erythropoietic Porphyria (Gunther Disease) DiGeorge Syndrome
Brain Tumors** Ewing Sarcoma* Neuroblastoma Ovarian Cancer* Renal Cell Carcinoma* Rhabdomyosarcoma Small Cell Lung Cancer* Testicular Cancer* Thymoma (Thymic Carcinoma)
Chronic Active Epstein Barr Evans Syndrome Multiple Sclerosis* Rheumatoid Arthritis* Systemic Lupus Erythematosus* Thymic Dysplasia
* in clinical trials, www. clinicaltrials.gov
Emerging Stem Cell Applications
Diabetes Heart Disease Liver Disease Muscular Dystrophy Parkinsons Disease Spinal cord injury Stroke |
Deciduous teeth
- Written by: Dr. Kouzi
Deciduous teeth

Biohellenika now, gives a second chance to children, who have not cryo-preserved their umbilical cord stem cells at birth, to preserve stem cells from their deciduous teeth that are replaced by the permanent teeth from the age of 5-6 years to 12 years. These cells are in experimental studies or clinical trials for regenerative medicine therapies.
Clinical Data
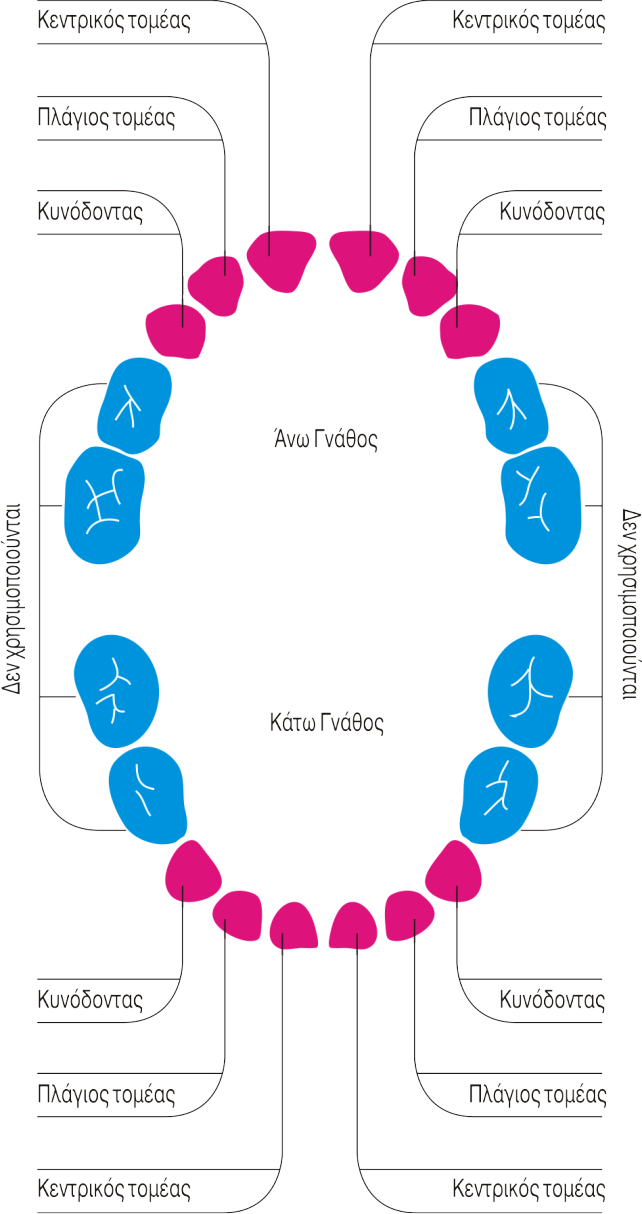
Latest clinical data indicate considerable potential use of stem cells of dental pulp.
Collection and Process

Stages collection, processing and cryo-preservation of dental pulp stem cells from deciduous teeth.
Pharmacogenomics
- Written by: Dr. Kouzi
Personalized Medicine
Biohellenika offers the opportunity for gene study of the patient, that will lead to personalized treatment, with more effective results and minimization of side effects.
Pharmacogenomics genetic tests offer not only information on the personal genetic structure but also prediction of the patient’s response to a specific medicine. Therefore, they can lead prescription of the most appropriate medicine in the most suitable dose. This is “personalized treatment”, which is specific, individual and ideal for the patient. There are genes that are specific for the metabolism of some drugs, resulting in the determination of the effectiveness of the treatment.
Home page BiohellenikaTV
- Written by: Dr. Kouzi
Biohellenika was successfully inspected by the American Association of |Blood Banks
- Written by: Dr. Koliakos
CORD BLOOD STEM CELLS ARE CLOSE ENOUGH TO REPLACE BLOOD DONATION
- Written by: Dr. Koliakos
New Publication of Biohellenika in PubMed
- Written by: Dr. Kouzi
Tests Table
- Written by: Dr. Kouzi
Offered Tests Table
GENETIC FACTORS FOR THROMBOPHILIA
Molecurar test of the genes: F5 (Factor V Leiden) (1691G>A) & 4044A>G)
F2 (Factor II) or prothrombin (G20210A)
MTHFR (C677T)
GENETIC FACTORS FOR CARDIOVASCULAR DISEASES
Molecular testing of 13 genes for 17 mutations:
- F5 (Factor V-Leiden) (2 mutations)
- F2 (Factor II) - prothrombin (1 mutation)
- MTHFR (2 mutations)
- F13b (Factor XIII) (1 mutation)
- FGB (1 mutation)
- ITGB3 ή GpIIIa ή HPA1 (1 mutation)
- PAI-1 (1 mutation)
- ACE (1 mutation)
- Apo B (1 mutation)
- ApoΕ (Ε2/Ε3/Ε4) haplotype study
- eNOS (2 mutations)
- LTA (1 mutation)
- PROCR (EPCR) (2 mutations)
GENETIC FACTORS FOR ATHEROSCLEROSIS
Molecular testing of 7 genes for 10 mutations:
- Apo B (1 mutation)
- ApoΕ (Ε2/Ε3/Ε4) haplotype study
- FGB (1 mutation)
- eNOS (2 mutations)
- ITGB3 ή GpIIIa ή HPA1 (1 mutation)
- ACE (1 mutation)
- LTA (1 mutation)
GENETIC FACTORS FOR THROMBOEMBOLISM
Molecular testing of 6 genes for 7 mutations:
- F5 (Factor V-Leiden) (2 mutations)
- F2 (Factor II) - prothrombin (1 mutation)
- MTHFR (2 mutations)
- F13b (Factor XIII) (1 mutation)
- PAI-1 (1 mutation)
- PROCR (EPCR) (2 mutations)
GENETIC FACTORS FOR ALZHEIMER DISEASE
Molecular testing of ApoE gene (Ε2/Ε3/Ε4)
IMMUNOGENETIC FACTORS FOR DISEASE PREDISPOSITION
Molecular testing of the allele of HLA Β-27
PHARMACOGENOMICS – PERSONALIZED TREATMENT
1.Molecular testing for the detection of genotypes in the DPD, associated with the patient’s response to treatment with 5-FU (5-fluorouracil): a) Allele 2 (IVS1G>A) – 1 polymorphism and b) Alleles *3, *4, *5A, *7, *8, *9, *10, *12, *13, Μ166V, R886Η, D949V.
2.Molecular testing for the detection of genotypes in the UDP gene, associated with the patient’s response to treatment with Irinotecan (or Camptosar).
3.Molecular testing for the detection of the alleles 1, 2, 3A, 3C in TMPT gene, associated with the patient’s response to treatment with sulfapyridine.
4.Molecular testing for the detection of the alleles *3, *4, *5, *6, *7, *8, *9, *14, *19 and *ΧΝ, associated with CYP2D6 gene, which metabolizes 25% of the prescribed drugs.
5.Molecular testing for the detection of 34 alleles in CYP2D6/CYP2C19 genes, that metabolize 40% of the prescribed drugs.
6.Molecular testing for the detection of the alleles *2, *2B, *3, *4, *5, *6, *7, *8, *9, *10, *11, associated with CYP2C19 gene, which metabolizes 15% of the prescribed drugs.
7.a) Molecular testing for the detection and identification of VCORC-1 (1639G>A) - 1 polymorphismand CYP2C9-2*(430C>T), CYP2C9-3*(1075A>C) – 2 polymorphisms, that are associated with the response to anti-coagulation treatment Warfarin and b) Molecular testing for the detection of the alleles *2, *3, *4, *5, *6, *11, associated with CYP2C9 gene, which metabolizes 5-10% of the prescribed drugs.
VIRUS MOLECULAR DETECTION
1. Qualitative detection of cytomegalovirus (CMV)
2. Quantitative detection of cytomegalovirus (CMV)
3. Qualitative detection of Epstein Barr Virus (EBV)
4. Detection of (Varicella Zoster virus -VZV)
5. Detection and typing of Herpes Simplex Virus 1 & 2 (HSV), using Real Time PCR
6. Qualitative and quantitative detection of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV), using Real Time PCR in serum
7. Detection and genotyping of 24 types of Human Papilloma Virus (HPV), using Microarrays.
MOLECULAR DETECTION OF BACTERIA, PARASITES AND GENITAL INFECTIONS
1. DNA detection of Mycoplasma hominis
2. DNA detection of Chlamydia trachomatis
3. DNA detection of Ureoplasma pavum/urealyticum
4. DNA detection of Group Β Streptococcus
5. DNA detection of Toxoplasma gondii
6. DNA detection of Mycoplasma genitalium
7. DNA detection of Neisseria gonorrhoeae
Molecular Analysis of Hereditary Diseases
- 1. Haemochromatosis (HH)
Molecular analysis of genes: HFE (12 mutations)
TFR2 (4 mutations)
FPN1 (2 mutations)
Also the HAMP gene can be tested and the whole sequence of HFE gene.
- 2. Cystic Fibrosis (CF)
- 3. Mediterranean Fever (FMF)
Molecular analysis of MEFV gene (12 mutations)
4. B-Thalasemia
5. A-Thalasemia (Hb-H)
Examples of additional single- and multi-factor diseases
- Angiotensin 1, AGT
- Hemorrhagic Telangiectasia Syndrome, I and II
- Hemophilia A and B
- Acatalasemia
- Amyotrophic lateral sclerosis
- alpha-1-antitrypsin deficiency
- Fructose intolerance
- Adenosine deaminase deficiency)
- 17-beta-hydroxysteroid-dehydrogenase 3 deficiency)
- 21-hydroxylase deficiency
- Ectodermal dysplasia
- Apolipoprotein B
- Apolipoprotein E
- Glycogen storage disease
- Αrrhythmogenic right ventricular dysplasia, familial
- Alzheimer disease
- Charcot-Marie-Tooth disease
- Fabry disease
- Gaucher disease
- Lou Gehrig disease
- Amyotrophic lateral sclerosis
- Niemann-Pick Disease
- Norrie disease
- Parkinson’ disease
- Pompe disease
- Tay Sachs disease
- Wilson disease
- Ataxia telangiectasia
- Achondroplasia
- Galactosaemia
- Glaucoma
- Dilated Cardiomyopathy
- Sickle cell anaemia)
- Systemic lupus erythematosus
- Cystinuria
- Cystinosis
- Lactose intolerance
- Xeroderma pigmentosum
- Retinitis Pigmentosa
- Metachromatic leucodystrophy
- Muscular dystrophy Duchenne/Becker
- Myotonia congenita
- Myotonic dystrophy
- Neurofibromatosis type 1and II
- Maple Syroup Urine Disease
- Spinal muscular atrophy
- Familial Mediterranean fever
- Familial hypercholesterolemia
- Τuberous sclerosis
- Homocystinuria
- Pancreatitis, hereditary
- Polycystic kidney disease
- Spinal and bulbar muscular atrophy
- APCR.
- Retinoblastoma
- Rheumatoid arthritis
- Diabetes, type I, II & MODY
- Severe combined immunodeficiency
- Angelman syndrome
- Bloom syndrome
- Gorlin-Goltz syndrome or Nevoid basal cell carcinoma syndrome = NBCCS or Basal Cell nevus Syndrome BCNS
- Lesch – Nyhan syndrome
- Louis Bar syndrome
- Lynch syndrome
- Nail - patella syndrome
- Prader Willi syndrome
- Rett syndrome
- Rubinstein-Taybi syndrome
- Sanfilippo C syndrome
- Smith-Lemli-Opitz syndrome
- Usher syndrome
- Von Hippel-Landau Syndrome
- Wolfram syndrome
- Tyrosinemia
- Hyperliproteinemia
- Adrenal hyperplasia, congenital due to 21 hydroxylase deficiency
- Hypertrophic cardiomyopathy
- Familial hypercholesterolemia
- Ectodermal dysplasia, Hypohidrotic or Anhidrotic, or Christ-Siemens-Touraine Syndrome, or CST syndrome
- Short stature
- Phenylketonuria
- Huntingtons disease
Stem Cells Collection
- Written by: Dr. Koliakos

Each child comes in the world with a gift from the nature, with a “life insurance”, with a quantity of “spare parts of cells” which if are preserved they will contribute for the health and its long life. (Roger Schort 2005)
Each child is endued with a supply of stem cells which can be used at some time for the safekeeping of its health, the improvement of quality or the extension of its life.
The birth of your child is a unique chance to preserve this valuable material. Do not take away from him/her this chance. The possibility to use the stem cells which are cryo-preserved at birth, nowadays it comes up to 1/400 for the child itself.
The use of stem cells apart from the child itself can also be extended to the parents, the siblings, as long as the tissue-compatibility is checked. So the up-to-date possibility for the graft to be used for a member of the family comes up to 1/200.
We have to notice that according to nowadays medical data the possibility which is bigger to 1/250 although is small can not be considered as negligible.
ASH Annual Meeting Abstracts) 2005 106: Abstract 1330
http://www.parentsguidecordblood.com/content/usa/medical/medmotiv.shtml?navid=20
This possibility has been calculated considering the nowadays approved applications without including the applications of regenerating medicine which are in the phase of clinical trials.
It is known that in the umbilical cord blood there is a category of cells which can be multiplied and transformed, so that they can replace cells of any tissue (Koegler et al 2003). The geometrical increase of the approved publications related to new applications admits the prediction of use all of the samples which are cryo-preserved.
For this reason our company thinks for sure that almost all the samples of the stem cells which are cryo-preserved today in liquid nitrogen one day in the future will be asked for use, consequently it takes care so that the thousands of children whose grafts are kept in our facilities, one day will be grateful to their parents for their precaution.
Biohellenika recommends to the expectant parents before they decide to keep the stem cells from the umbilical cord blood and before they choose the company for the cryo-preservation of the valuable material, to visit for their information, independent web-sites especially the ones which were created by parents or unions of parents abroad such as: www.parentsguidecordblood.com and www.cordblooddonor.org.
Read about pro and con of the umbilical cord blood preservation in:
http://www.parentsguidecordblood.com/content/usa/medical/medmotiv.shtml?navid=20
The Cord Blood forum (http://www.cordbloodforum.org/index_flash.html) contains information of the up-to-date medical application of the cells which are coming from the umbilical cord blood.
The umbilical cord blood contains the biggest quantity of any other source of stem cells, can be collected at the natural birth but at the cesarean too, and it is once in a lifetime opportunity to collect these cells. The procedure for the collection begins just after the end of the labor and the cut of the umbilical cord, so it can do no harm mother or the baby. The collection of the umbilical cord blood is performed by puncturing the umbilical cord after local antisepsis. From this blood stem cells are separated, frozen and kept in liquid nitrogen conditions in minus 196˚ C indefinitely.
The stem cells can be used for the therapy of over 50 diseases.
Real Stories
- Written by: Dr. Koliakos
THE REAL STORY OF LITTLE ATHANASIOS
Little Athanasios was born after a very difficult and long natural labour, because he was overweight. The middle cerebral artery was thromboses and his brain was deprived of oxygen for a long time. This condition led to cerebral palsy. He was the forth child of the family and the family, who wanted to help all its children, decided to cryopreserve Athanasios umbilical cord blood, just in case. At that moment they did not realise that one day these cells would be very helpful for someone in the family. Soon after his birth, Athanasios presented signs of cerebral palsy and the family started treatment using all the known methods. At age of 20 months his mother was informed that a child with the same as Athanasios’ problems was treated in the USA by using its own stem cells that her family had preserved during her birth. The mother remembered that she had cryopreserved Athanasios cells and asked Biohellenika if Athanasios could be a candidate to take his cells. Since then a long procedure started: Biohellenika and Duke University exchanged information about stem cells banking and medical records. Finally after making all the preliminary tests the family and the cells were transferred to Duke University for the infusion. The family stayed in the USA for 4 days and Athanasios was infused with his own stem cells. The procedure was executed safely and without any side-effect. Later Athanasios came back and reported regularly to the neurologists that took care of him during his stay in Duke University. The stem cells were cryopreserved in 3 cryovials. All the quality controls after post-thaw were done in Biohellenika’s labs. The viability of the cells was 96% and for this reason Athanasios took only the cells from two of the cryovials. The third remains in Duke University for future use in case it is needed. Having experience from the first child treated, we expect improvement after three months.
The University of Georgia announced that it will start a clinical trial with 40 children with cerebral palsy that have cryopreserved their own stem cells in collaboration with a private cord blood bank. The trial will include children until 8 years old that already have cryopreserved umbilical cord blood stem cells. At the moment the treatment includes only autologous use of stem cells, even in the case of fully matched sibling donor.
Danae’s real story.
Autologous administration of stem cells, stored by Biohellenika for the treatment of Danae who suffered from cerebral palsy.
Biohellenika administered an umbilical cold blood stem cell sample to a 31 month old child suffering from cerebral palsy. This autologous administration is a unique success for Biohellenika, the largest Family Bank in Greece, since it is the first unit of cord blood that has been cryopreserved in a Greek bank and administered successfully from a University Hematology clinic of USA.
The cerebral palsy was caused due to complications during birth, resulting to the insufficient oxygen supply to Danae’s brain for sometime and the baby presented problems in muscle tone and strength during the first months of its life. The family addressed Biohellenika directly and the head of the scientific board Dr. Kouzi-Koliakou communicated with the Hematology clinic of Duke University USA.
Biohellenika sent the complete file of cryopreservation of the baby’s placenta blood to the clinic. The file was examined closely and approved by the doctors of the clinic. Then, the medical team requested the transport of the child and the sample. The initial blood collection by the obstetrician was enough, although it was a twin birth. This had as a result the number of the cryopreserved stem cells to be sufficient for transplantation. The stem cells administered to the child were analogous to a double of the weight the child had. In autologous use the administration of a large number of stem cells is useful for the patient, as it has a better therapeutic outcome and there is no fear of rejection, as the child uses its own stem cells that are completely compatible.
The stem cells were sent in a special transfer container that is called Voyager. The stem cells can safely remain in the Voyager up to 23 days, a period which is sufficient for transport to any place of the world.
The stem cells were cryopreserved in five cryo-vials. Two of them contained a smaller volume for quality tests before the thawing. The total number of stem cells the vial contained was more than enough.
The administration of the stem cells was scheduled for 28/7/09. A day before the transplantation the child was examined by a neurologists’ team that assessed and logged all clinical findings for future reference. All cryo-vials were thawed and the cells were administered to the child intravenous and under the surveillance of all its vital signs. The child did not present any complications during the administration of the cells that lasted 10 minutes. Immediately after the transplantation, the family left the hospital and came back the following day for a new check up from the doctors. The following day they left the hospital and today they are in Greece. Continuous improvement in the movement, speech and communication is noted every day. The child remained in USA only three days, treated as outpatient and didn’t pass any night in the hospital.
According to the experience of the doctors a significant improvement of the movement, decrease of the muscle spasticity and improvement in communication is expected after the third month. Up to day the child has presented clear signs of improvement as she is now able to speech more fluently, communicate with her family, stay and walk and generally she has more confidence in starting and finishing actions.
The administration of the cells was safe and no side effects were noticed to Danae. Biohellenika cryopreserves clear nucleated cells, after almost full depletion of red blood cells, so the amount of cryoprotectant DMSO is very low. Because of that the side effects like vomiting, nausea and changes in the blood pressure are very rare. The child during the whole procedure was completely calm and cheerful.
Larger volume of the cryopreserved sample, needs larger amount of the cryo-protectant DMSO, thus the side effects are stronger. In Biohellenika the stem cells are cryopreserved in cryo-vials were a pure population of stem cells is stored, without impurities of red blood cells. This has as a result a smaller cryopreserved volume and therefore no unwanted side effects to the patients during administration.
The opportunity given to this family is due to the mother’s foresight, who persisted on new year’s eve to receive Biohellenika’s collection kit by any means, to the doctor’s conscientiousness who performed two big cord blood collections from the babies that were twins and born with a cesarean, something very difficult many times, and to Biohellenika’s personnel that on new year’s eve processed with the best possible way a sample that justified every ones efforts. All data lab obtained after the thawing of the sample in the laboratories of the Transplantation Unit of Dukes University showed a large number of cells of high grade of survival, clear from bacteria and viruses and with an intense capability of proliferation. The chances of improvement of the young patient are very high. Up until today Biohellenika has administered with success more than 30 samples to patients for different diseases.
On 26th of January another child with the same problem will take the same way for treatment.
DALLAS HEXTELL

Stem cells from Dallas umbilical cord blood were the only hope for the family, stated Cynthia Hextell, Dallas mother, who was suffered from cerebral palsy since birth and transplanted with its own stem cells. The family never forgets the day that Dallas was diagnosed with cerebral palsy. It was tragic, Dallas did not even sit nor had sight and the only way for him to communicate was endless crying. Soon after birth the parents realized that something was wrong with Dallas. Fortunately Dextells had cryopreserved Dallas umbilical cord blood stem cells, for just in case and they never believed that they will be used in the future, because of Dallas sickness. Their decision then was proved valuable and wised today. On the age of 18 months old Dallas received its cells by a simple procedure. Retells did not know what they will expect from this treatment, but fortunately they did not wait for along. Dallas therapist, Polly Harlan noticed serious changes soon after the transplantation. The first signs of Dallas improvement were the participation in family’s life, fillings expression and speech development. Lately he starts to stand, walk smile and be happy. Moreover he became a real boy. For Cynthia it was a miracle. The results were so impressive and fast and stem cells gave to Dallas and his family the opportunity for a real life.
Doctors now are looking to expand the therapeutic applications of stem cells to others cases like Dallas. According to Dr David Harris, Professor of Immunology in Arizona University “ stem cells from the umbilical cord blood is the new hope for the children with cerebral palsy, since they have the ability to insert into brain’’ Retells family is grateful for their decision to bank Dallas stem cells, since this action gave them a hope for Dallas future. In cerebral palsy, as a result of shortage in oxygen, the child’s umbilical cord blood stem cells can improve its life, when they were infused during the first two years of life.
Hextells family wants to inform the future parents for the benefits of stem cells banking, because nobody knows when they can be used
KEEGAN & KELDAN D0HENEY

Your son suffers from ‘’leukaemia‘’. It is very painful for somebody to hear this diagnosis, but Wendy Doheney had to hear two times. The first time Keegan was just two years old. Doctors initially put Keegan’s leukaemia in remission, and Doheneys were a little happy. The second time was three years later. ‘’Keegan was in school, kindergarten, and me in home’’ Wendy remembers. ‘’And suddenly I had this filling, that something was going wrong. I called the Keegan’s doctor, expressed my filling and she thought that I lost my mind. There were no sign of wondering, but just simply I knew. Unfortunately Wendy’s instinct was proved right and keegan’s leukaemia return. Fortunately this time Doheneys had cryopreserved the little brother’s Keldan umbilical cord stem cell. If siblings were histocompatible, then Keldan’s stem cells would be use to treat Keegan’s leukaemia. ‘’We were so relieved, because we banked Keldan’s stem cells’’ Wendy remembered. ‘’When I was pregnant we had be informed about stem cells and all the ‘’miracles’’ that happen by using them. We examine all the possibilities and we decided to bank, for just in case. The good news came very soon. The boys were compatible. The doctors transplanted Keldan’s stem cells to Keegan body and now Keegan is healthy, more than nine years. An excellent student and athlete, Keegan has a very special bond to his little brother Keldan. ‘’ My little brother saved my life’’ said. Because of him now I am here, alive.
 When Chloe Levine was 9-months-old, her parents noticed she couldn’t hold her bottle with her right hand.
When Chloe Levine was 9-months-old, her parents noticed she couldn’t hold her bottle with her right hand.
That wasn’t her only developmental setback. Chloe, of Pinetop, Ariz., was unable to raise both hands above her head, and she could not crawl.
At 12 months, a CAT scan showed a portion of the left side of Chloe’s brain had not developed and contained fluid. Seeking answers, Chloe’s parents, Ryan and Jenny Levine took their daughter to a neurologist who diagnosed the toddler with right-side hemiplegic cerebral palsy.
“The cerebral palsy had only affected the right side of her body,” Jenny Levine said Monday morning on FOX & Friends. “The neurologist told us we were looking at 17, 18 years of therapy.”Click here to watch Dr. Manny Alvarez, managing health editor of FOXNews.com, speak with the Levines on FOX & Friends.That was when the Levines heard about an experimental procedure at Duke University in North Carolina where children with cerebral palsy were infused with their own cord blood stem cells in an effort to heal and repair damaged brain tissue.The Levine’s remembered they had banked Chole’s cord blood when she was born.“It was a miracle,” Alvarez said Monday on FOX & Friends. “I congratulate you for banking her cord blood. Stem cells are a new field of medicine and they certainly can rejuvenate the tissue.”Two months ago, Chloe, 2, received an infusion of her own stem cells and her progress is remarkable, said her father, Ryan Levine.“Her therapist said she’s made a 50 percent recovery,” he said. “She can walk, run, and do sign language with her right hand.”“It’s a miracle,” agreed Jenny Levine. “To hear your baby’s voice is a gift.”Alvarez said all parents expecting babies should consider cord blood banking.“There is no downside, this is material we used to throw away,” he said. “And while the blood is most useful for the child it came from, it can sometimes be used for siblings. This is a science that is evolving more and more.”Storing cord blood costs about $2,000, but Alvarez said he expects the price to come down in the near future. Cerebral palsy refers to any one of a number of neurological disorders that appear in infancy or early childhood and permanently affect body movement and muscle coordination but don’t worsen over time, according to the National Institute of Neurological Disorders and Stroke. It is caused by abnormalities in the parts of the brain that control muscle movements.
Questions before decisions
- Written by: Dr. Koliakos
Because the choice of a company for collection and storage of your stem cells is a serious decision, to make it easier for you, we have written the following important questions before your final choice.
- Has the bank been certified and inspected from a public authority?
- Does the company work legally in Greece?
- Does it collaborate with a hospital or a big research center?
- The process, the quality controls and the storage of the sample is performed in the company’s own laboratories?
- The equipment that are used in the laboratories are specially designed for the use of stem cell samples or are they used to perform other medical tests or other cells storage?
- The reagents and the equipment that are used have the label CE and are they designed exclusively for human use?
- Have stem cells that have been stored by the bank been used for a transplantation and what was the result?
- What happens in the case of sample’s contamination or small volume?
- Is the collection kit of the sample easy and safe to use from the obstetrician/nurse? Are they prepared in the case of premature birth?
- What measures have been taken in the case of company’s bankruptcy?
- Is the staff of the company available any time to answer all your queries or solve any problem before, during, or after the collection of the sample?
- is the cost of the process-storage of the sample within logical bound? Is there the possibility of a financial settlement?
- Is the cost of the services stable the next 20 years or there is a possibility to rise?
- In the case of inability of the parents to pay directly the company, what will be the consequences for the sample and the process of its storage?
- In case of twin birth or when the storage is performed for a second child of the family is there any discount?
- How the company guarantees the safe transport of the sample from the hospital to the laboratories and how long it is take?
- Does the company provide support to the parents in the case of illness of the child from a serious disease that could be treated with the stored stem cells?
- Is there a guidance in case of illness and are the parents constantly informed for the new areas if stem cell applications?
REASONS TO CHOOSE BIOHELLENIKA FOR YOUR BABY STEM CELLS COLLECTION AND PRESERVATION
- Biohellenika is an AABB (American Association of Blood Banks) accredited company that ensures the quality and the acceptance of stored cells in hospitals all over the world for autologous or family treatment.
- It is the first private company in Greece that is licensed by the National Transplant Organization
- Biohellenika is accredited by the National Accreditation System (ESYD) for all tests that must be done in stem cells and mother’s blood for a successful cryopreservation and treatment.
- Biohellenika provides double storage facilities in its labs in Athens and Thessaloniki for safety reasons and for emergency treatments. So every patient can reach his/her sample immediately.
- Proceeds the whole length of umbilical cord and give the clients the highest number of mesenchymal stem cells, ready to use. This procedure is protected by the patent No 1007490. According to this method there is no need of cell proliferation, to increase the number of cells, a procedure that is not permitted at first by the law, needs time to be accomplished, it is not safe and the quality of cells after proliferation is different than the initial.
- Biohellenika does not cryopreserve the umbilical cord in pieces, but at first extract the cells from the total length and then preserve the cells in more vials, ready to use up on the request.
- Provide a second selection of hematopoietic stem cells directly from placenta after perfusion, a method that is protected by the patent No 1007478). By this second selection a double number of haematopoietic stem cells stored that is enough to treat overweight patients with malignant diseases and also for more than one use.
- Biohellenika uses an AABB accredited Traceability system, ISBT 128, that follows the sample after enrolment and ensures a safe treatment.
- All samples are tested for microbe contamination and in case of detection the cells are treated with the appropriate antibiotics.
- All cord blood samples and mother’s blood are tested for virus contamination of hepatitis B, C, HIV and CMV by PCR method and the mother it is not necessary to repeat these tests six months later.
- Biohellenika inform parents after procedure with the appropriate medical document that describes the total number of nucleated cells, the haematopoietic and mesenchymal stem cells, by flow cytometry, microbiology and virology tests.
- Biohellenika pryopreserves the stem cells in multiple cryovials, integrally attached, system protected by the patent 1007483, that permits each time to use the appropriate number of cells.
- 805 autologous treatments with the stem cells that are stored in Biohellenika’s labs have been successfully completed up to now.
- According to article 3 of the Private Agreement that signed by Biohellenika and parents for the 20 years storage, Biohellenika provides the cells in 48 hours after a written request.
- Biohellenika provides free medical consultation until the expiration date of the Private Agreement by a highest quality team of Academic Doctors.
Adipose Tissue
- Written by: Dr. Koliakos
 Adipose Tissue
Adipose Tissue
Biohellenika the greatest company of stem cells storage in
Studies have shown that stem cells that we have in our body can lose some of their potential as we grow older. As the need for use in regenerative medicine is increased, it is necessary to preserve our cells as we are young and healthy. The doctors can aspirate adipose tissue either during a visit as an exterior patient, of during a scheduled liposuction.
Biohellenika will store the stem cells with safety and deliver them ready to use, after thaw, when the patient’s doctor will decide to use them.
New clinical trials use the stem cells of the adipose tissue for many therapeutic applications, including cardiovascular diseases, applications in plastic and esthetic surgery, skeletal regeneration and gastrointestinal tract malfunctions.
Analytically, the storage of stem cells from adipose tissue is now important, and they can be used immediately, without side effects:
1. Because now our cells are young, vital and free from any disease, probably they may not be as healthy when they are needed.
- Because our valuable cells must not be disposed after liposuction or any other surgical procedure
Adipose tissue derived stem cells already have been used in:
Breast reconstruction after partial mastectomy or lumpectomy. Also in combination with lipotransferring for breast augmentation
Tissue regeneration after irradiation necrosis
Calvarial repair after injury
Fistula healing: tracheal (tissue necrosis), perianal and Crohn’s disease
Acute GVHD after bone marrow transplantation
Facial and skin rejuvenation and other cosmetic corrections of the skin that are arose from aging and sun exposure
Experimental studies that are shown promising therapeutic use in the near future:
Multiple Sclerosis
Hemorrhagic or ischemic stroke
Spinal cord injury
Parkinson’s disease
Pulmonary disease
Urinary incontinence
Acute renal tubular disease after chemotherapy
Osteoarthritis
Liver disease
Diabetes I
Acute or chronic cardiac ischemia
Diabetic ulcers and peripheral arterial vascular disease
More promising therapeutic uses:
Intervertebral disc repair
Muscle, bone or tendons repair after injury
Vocal fold repair
Corneal repair
Periodontal disease
Families
trusted us
The first
therapeutic application of stem cells in Greece was made by us
Years
we are the first in quality and stem cells applications
Publications
in scientific community
Patents
Worldwide