Τι πρέπει να γνωρίζω
Μάθετε για τις χρήσεις των βλαστοκυττάρων
Ενημερωτικό Υλικό
Μάθετε για τις χρήσεις των βλαστοκυττάρων
Κλείστε ραντεβού εδώ
Ενημερωθείτε υπεύθυνα για τα βλαστοκύτταρα
Offices
For the parents service the offices of Biohellenika are open in Thessaloniki in ZEDA building daily from 9 am till 9 pm and on Saturday from 9 am till 4 pm. Also, an office at 137 Tsimiski street on the 6th floor, is open daily 5-9pm and the rest of the day if it is asked so. Tel 2310 474282-4
For the South Greece families’ service, offices of the company are open in Athens, Archelaou 28A Pagrati. Furthermore, for the service of the families who live in Thessaly offices are open in the central square of Larissa, 23 M. Alexandrou str., on the 6th floor, tel 2410-535603, office in Patras Kos and Panepistimiou 3, thl 2610437436, Iraklion Amalthias and Katexaki tel 6970803497. Ioannina Park of Technology, Ioannina tel 2651097667, 6970267540, 6949441906
Information
The parents can be informed for the provided service, the potentiality of its application and can discuss the need of the service use based on the medical history of the family. The information is given by responsible scientists of the company, or after an appointment, by a medical professor of the Medical School.
The parents, having an appointment, can also visit the labs of the company and have a tour by a special scientist.
Receipt of the collection package
The parents receive by our offices without any charge the bag for the collection of the umbilical cord blood (collection bag) as well as the documents accompanying it. The parents, having no obligation, receive the contract in order to study it.
The collection bag for the umbilical cord blood is followed by the strictest national specifications so that the quality of the sample is secured as long as it is transported.
The collection bag for the umbilical cord blood, the documents which accompany it and the contract may be delivered at home or they can be posted to you with company’s expenses.
Financial agreement and signing of the contract
The parents are to pay or to agree on the way they want to pay and to sign the contract only after the successful cryo-preservation and the fulfilling of the quality control.
The Biohellenika contract has been done to protect the children and the parents from any possible danger of the sample loss and it improves itself all the time for the clients’ benefit. Every improvement and additional right or ensuring concerns retroactively all the prior clients.
Biohellenika gives priority to the quality and the best service of its clients. For this reason it makes sure, as far as it is possible, to keep the price of the service affordable for the family. Taking that under consideration we have made agreements with banks, to make it easy for the parents to pay in full with 12 installments without interest.
The sample’s collection
Biohellenika has achieved the minimum collection time for the blood samples in Thessaloniki, Athens and Larisssa 1 hour and for the rest of Greece 7 hours. Biohellenika respecting the choice and the the parents to shield their children’s health has its labs in daily function during holidays and furthermore, in two rolling shifts.
In Thessaloniki, Athens, Patra, Larissa, Heraklio and Chania the collection bag is taken immediately from the obstetric clinics by a representative of our company. In other cities the company collects the bags, with its responsibility, from the drivers of the public transport means and informs the parents at once for the collection of the sample and the safe arrival to our labs. It is also parents’ choice the use of a specialized company. For the Aegean islands, the company is cooperating with two transportation companies which are specialized on the transportation of medical samples.
A study of the Scientific Team of Biohellenika which refers to the effect of the conditions and the time of the umbilical cord blood transportation from the obstetric clinic to the lab, concerning the viability of the stem cells, is about to be published in the international scientific Journal Transfusion, official Journal of the American Association of Blood Banks (AABB). In this publication it is proved that the within 12-hours cryo-preservation and until then the preservation stay at 4˚C, constitute the conditions which assure excellent viability of the stem cells.

Umbilical cord blood: (Classic puncture of umbilical cord blood vessels)
The collection of blood is made by your obstetrician immediately after labor. The proper collection of blood is very important for the quality and quantity of the cell.
The process is simple, takes about 5 minutes and is completely safe for the child and for the mother, (as it occurs after birth). Parents must take on time the proper kit of Biohellenika, which must remain in perfect condition until transferring to the labour room on the day of delivery. The kit is packaged in isothermal condition with a registration of temperature to keep the cord blood at a stable temperature.
Cord blood is collected in a special sterile bag and sent to laboratories of Biohellenika, where there is going to be done all the controls and the cryo – preservation of stem cells. We have observed that the collection of stem cells is different in every birth and for this reason it is absolutely necessary to collect the maximum amount of blood. In case of twins-multiple pregnancy, the volume collected from each child is smaller, but this does not cause problems for further use. All data so far, indicate that the age, maternal ethnicity, weight taken by mother during the pregnancy and sex of the child, did not play any role, positive or negative, on the successful collection of cord blood. The things that seems to have result in receiving a higher volume of blood, is the child’s weight at birth and the completion time of pregnancy. Usually, as larger volume of blood collected, as higher the final number of stem cell frozen. Forthwith, the specific isolation method used by Biohellenika, can process small volumes samples. So, Biohellenika’s final acceptance test of the sample is the number of cells in the sample and not the original volume of it.

Bibliography
• Ljungman et al, Bone Marrow Transplant 2006, 37: 439-49
• Strauer et al, J Am Coll Cardiol, 2005, 46: 1659-61
• Sanberg et al, Ann NY Acad Sci 2006, 1049: 67-83
• Ende et al, J Med, 2001, 32: 241-7
• Rocha et al. Transplants of umbilical cοrd blood or bone marrow from unrelated donors in adults with acute leukemia. Ν Engl J Med 2004, 25; 351: 2276-85
• Locatelli et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood 2005, 105; 1: 410-419
• Kato et al. Cord blood transplantation from sibling donors in Japan. Report of the national survey. Int J Hematol 1998, 67: 389-96
• Varadi et al. Human umbilical cord blood for hematopoietic progenitor cells transplantation. Leuk Lymphoma 1995, 20: 51-58
• Kobylka et al. Preservation of immunological and colony-forming capacities of long-term (15 years) cryopreserved cord blood cells. Transplantation, 1998; 65(9): 1275-1278
• Eapen et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol 2006, 24: 145-151
• Wiley and Kuller. Storage of newborn stem cells for future use. Οbstetrics and Gynecology Vol 1997, 89 (2): 300-303
• Gutman et al. Autologous transplantation followed closely by reduced-intensity allogeneic transplantation as consolidative immunotherapy in advanced lymphoma patients: a feasibility study. Bone Marrow Transplant 2005, 36; 443-51
• Lychtenstein et al, New England Journal of Medicine 2000: 343 (2): 78-85
• Moezz et al. The effect of cryopreservation on clonogenic capacity and in vitro expansion potential of umbilical cord blood progenitor cells. Transplant Proc 2005, 37 (10): 4500-3
• Sartor et l. Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products. Bone Marrow Transplant, 2005 36: 199-20
Cytological control
All photographs are provided by the scientific team of Biohellenika.
Τhree types of measurements are performed before and after the procedure of stem cells separation
1. White and red blood cells count with the certified for clinical use Beckman Coulter (CE-IVD)
2. Viability and number of haematopoietic stem cells (HPC) CD45dimCD34+, according to the ISHAGE protocol by the flow cytometer Epics XM Beckman Coulter
3. Culture of a small number of haematopoietic cells in a specific medium for colony forming BFU-E CFU-G and CFU-GM.
Cell Types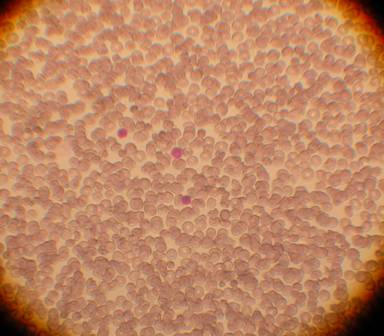
Image 1. The total blood as first coming in the lab, before processing. We can see three nucleated cells and the rest red blood cells. Red blood cells are not stem cells. Stem cells are included in the nucleated population
The population of leukocytes in the umbilical cord blood contains 2 types of cells: the haemopoietic population of CD45dim/ CD34+ cells and the population of multipotent CD45-/CD34 cells. VSELs are included in this population. Both populations show great differences in their morphology and their development. The haemopoietic population comprises of more mature cells which constitute the population that will be transformed to haemopoietic cells after transplantation (Images 2 & 3).
|
|
|
| Image 2 Homogenic population of nucleated cells of the umbilical cord blood after enrichment. Note the total absence of red blood cells after the completion of Biohellenika’s isolation protocol |
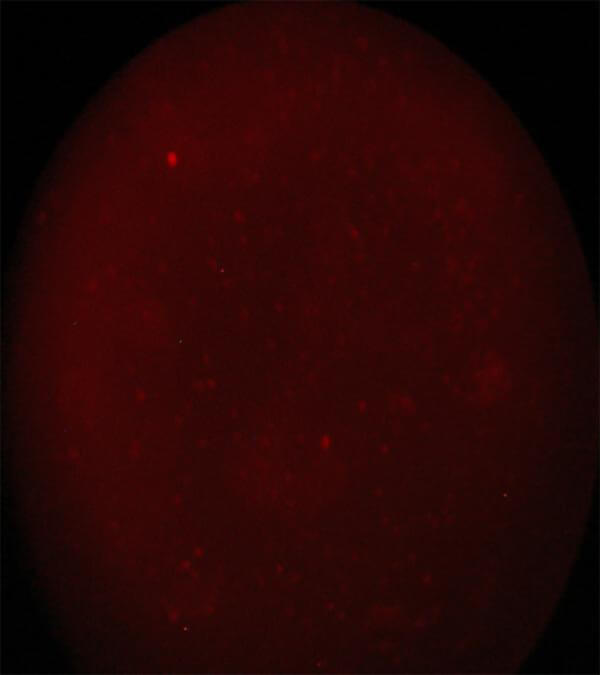
Image 3 CD 45+ cells. Immunofluorescence method using a reverse microscope Zeiss AXIONVERT 40 CFL |
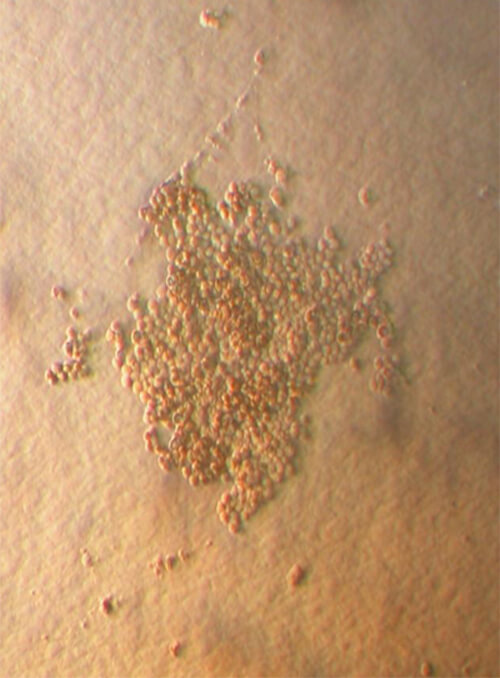
The population of multipotent cells consists of spindle type cells with extensions resembling fibroblasts, and is characterized by the ability to divide 40 times without further differentiation, reaching a population of 1,015 cells without karyotype changes (Gogler et al, 2004, 200: 123-135). This population is a subject of intensive research all over the world and today this type of cells can differentiate in neurons, osteocytes, chondrocytes, fat, cardiac cells, muscular and hepatic cells (See Image 4).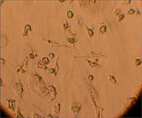
Image 4
Appearance of multipotent stem cells of fibroblast type
in umbilical cord blood after culture for 15 days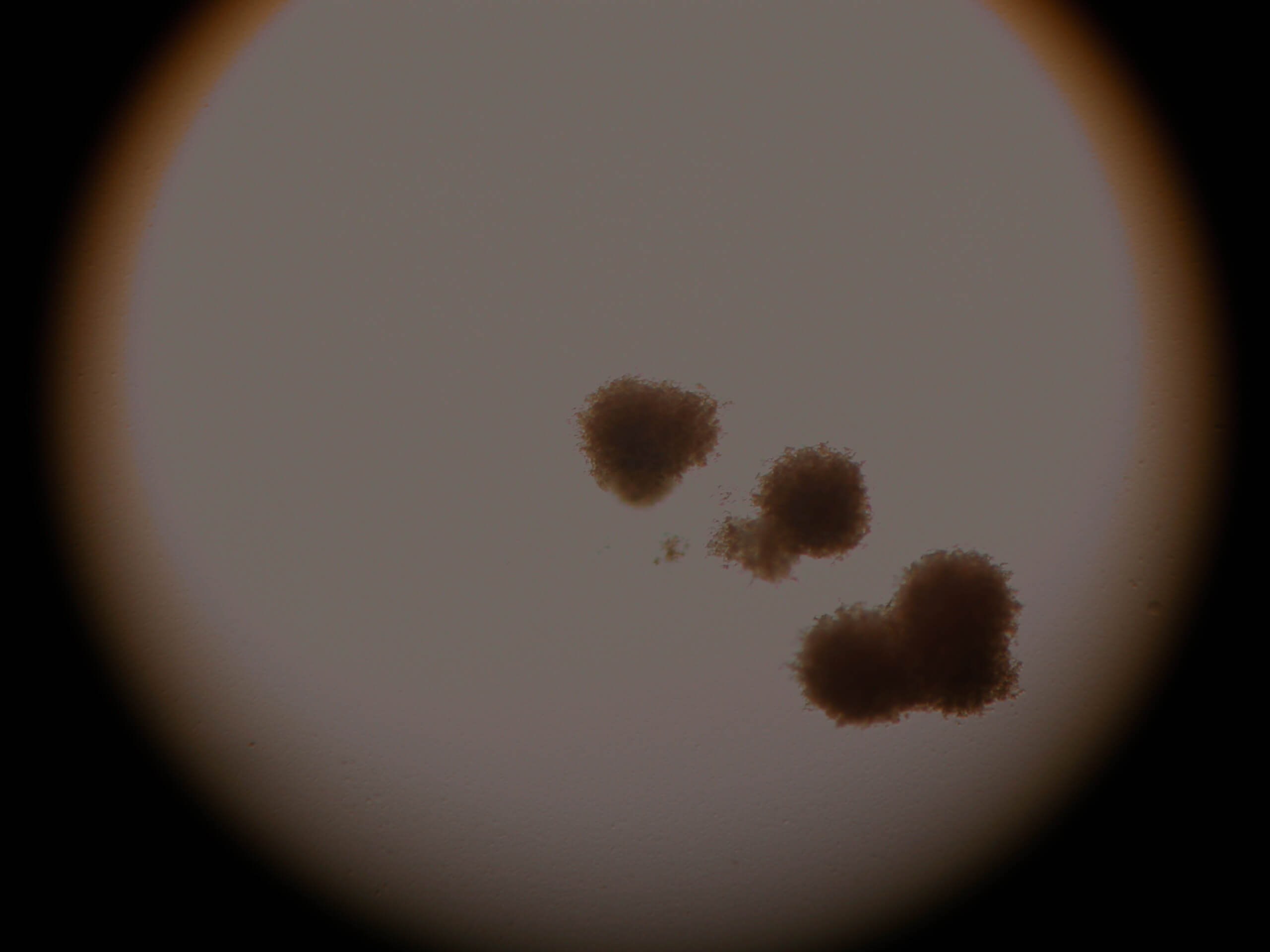
Image 5. CFU of red blood cells. Stem cells maintain all the biological abilities.
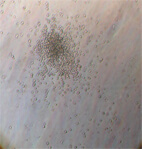 |
 |
| Image 6 | Image 7 |
Images 6-7. CFU of neutrofil-granulocytes-macrophages
Therefore, the CD34+ proliferation for the increase the number of stem cells for haemopeisis and to treat leukaemia is not in use. Today small molecules (like nicotinamide) are tested for CD34+ expantion succesfully. The number of stem cells required for the treatment of leukaemias depends on the body weight of the recipient. The number of nucleated cells from umbilical cord blood reaching 1.73x107 /Kg body weight and the number of CD34+ cells reaching 2.7x105 /Kg body weight, have been administered successfully to patients with leukaemias (Ren et al 2001, Zhonghua, 22: 621, Cilley et al 2004, Bone Marrow Transplantation, 33: 161-4, Magro et al 2006, Hematologica, 91: 640-8). The number of stem cells is proportional to the total volume of collected blood. However, due to the presence of multipotent cells that allow proliferation, even small quantities of blood can nowadays be cryopreserved.
Virology control
Virology tests l for hepatitis B,C,HIV and CMV in the umbilical cord blood with the method RT.PCR.
RT.PCR can detect immediately the virus load in child’s blood and relieves mothers from continuous examination during and after pregnancy. Recently Biohellenika was equiped with Compass instrument by Roche for the direct virus detection in the babys umbilical cord blood.
Bacteriology control
Bacterial aerobious and anaerobious control in three certified for clinical use (CE-IVD) Bactec systems (Becton Dickinson) of total capacity of 290 samples. These systems are characterized by their great sensitivity and reliability of the results.
3-12% of umbilical cord blood units worldwide are considered unsuitable due to contamination. According to our experience in private obstetrics clinics is nearly (0.5-1%) due to special care and skill of obstetricians.
In case of positive bacteriological control, the sample might not be suitable for use but only after the bacterial characterization and the antibiotics and therefore is cryo-preserved for free.
Results of the quality check
The parents receive the medical results of the quality control signed by the doctor in charge (university professor).
All the above mentioned quality methods are accredited by AABB and the Hellenic Accreditation System according to 410, 8/2/2008 published document.
 Bone Marrow
Bone Marrow
Biohellenika selects bone marrow stem cells after aspiration of the iliac crest from patients that are undergone coronary by-pass. The cells after isolation in Biohellenika’s labs are injected directly to the cardiac muscle at the periphery of the infarct in the end of the surgery. The procedure is performed due to prior myocardiac infarction or chronic ischemic myocardiopathy. The stem cells usually develop angiogenesis in the next few months and rescue the "hibernating" myocardium. Clinical trials show that stem cells can improve cardiac function shortly after the administration.
Stem Cells Bank Biohellenika - Web design Hexabit - W3C - Pagespeed